Introduction
A major challenge in allogeneic hematopoietic cell transplantation (HCT) has been advancing outcomes for individuals lacking a fully matched donor who require a mismatched unrelated donor (MMUD) HCT. Recently, the 15-MMUD study demonstrated encouraging results for post-transplant cyclophosphamide (PT-Cy)-based prophylaxis in MMUD recipients (Shaw, Jimenez, JCO 2021). Our retrospective analysis confirmed improvement in MMUD survival with PT-Cy compared to historical anti-thymocyte globulin (ATG)/calcineurin-based regimens (Jimenez, Blood Adv 2022). Elevations of plasma biomarkers such as Stimulation-2 (ST2) have demonstrated increased risk of non-relapse mortality (NRM) and GVHD, however these have not been validated in the MMUD setting. Here, we carried out a pilot study to determine if biomarker analysis of ST2, Chemokine ligand-9 (CXCL-9), and Regenerating islet-derived 3-α (REG3α) at Day 14 (D14) and 30 (D30) can identify patients at higher risk for NRM after MMUD HCT.
Methods
Patients with banked samples and underwent MMUD HCT with either ATG or PT-Cy as GVHD prophylaxis at the University of Miami between 2016 - 2020 were included. Enzyme-linked immunosorbent assays for ST2, CXCL-9, and REG3α were performed in batches on cryopreserved plasma. Descriptive statistics of demographic variables were compared using chi-squared, Fisher's exact, or Wilcoxon rank sum tests. ST2 values were treated as continuous variables, and CXCL-9 was dichotomized due to frequent values below the lower limit of detection. Cumulative incidences of NRM were analyzed with relapse as a competing risk. Time-dependent receiver operating curves (ROC) were constructed and Area Under the ROC Curve (AUC) calculated. Youden's index was used to select optimum thresholds for each biomarker.
Results
We included 47 patients (ATG: 23; PT-Cy: 24). The PT-Cy group had more bone marrow graft representation (50% vs 22%, p = 0.04) and more donors mismatched at >1 HLA-locus (29% vs 0%, p<0.01). Median follow up was 746 days (range 44-1968). Point estimate of overall survival at 2 years was 35% (95% CI 20-61) for ATG and 71% (95% CI 55-92) for PT-Cy (p=0.02). Median overall survival was estimated at 413 days among ATG patients and could not be estimated for PT-Cy patients. The cumulative incidence of NRM at 2-years was 30% (95% CI 13-51%) for ATG and 17% (95% CI 5-35%) for PT-Cy (p=0.03).
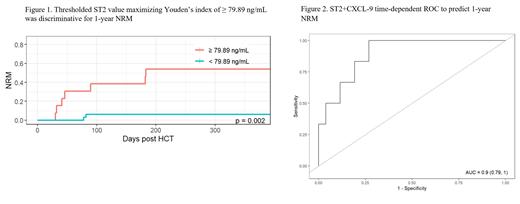
Among all patients, ST2 was prognostic for NRM using time-varying covariates including combined D14 and D30 (D14+30) with a HR of 2.14 (95% CI 1.04-4.39, p = 0.04). The D14+30 ROC to predict NRM at 1-year using ST2 showed an AUC of 0.83 (95% CI 0.66-1). D+14 thresholded ST2 value maximizing Youden's index of ≥79.89 ng/mL was discriminative for 1-year NRM (p=0.002) (Fig. 1).
Additionally, combination of CXCL-9 to ST2 could optimize the prognostic value when compared to ST2 alone: HR at D14+D30: 3.82 (95% CI 1.01-14.47, p=0.048) with an improved AUC of 0.9 (95% CI 0.79-1) (Fig. 2). REG3α did not significantly add to the AUC.
Although limited by sample size, we explored the association by GVHD prophylaxis (ATG or PT-Cy). First, in the ATG group, the D14 ST2 value that maximized thresholded Youden's index of 61.39 ng/mL was predictive of 1-year NRM (p=0.04). Similarly, the D+30 ST2 values of 148.06 ng/mL was predictive of NRM (p<0.001). The AUC for prediction of 1-year NRM for
Second, in the PT-Cy group, elevations of D30 ST2 were prognostic of 1-year NRM with HR 15.05 (95% CI 1.77-27.83, p=0.01). Consequently, the AUC for prediction of 1-year NRM for D30 ST2 was 0.92 (95% CI 0.77-1) and for ST2 + CXCL-9 was 0.96 (95% CI 0.86-1).
Conclusions
This pilot study suggests that after MMUD HCT, D14 and D30 biomarkers are prognostic for NRM across GVHD prophylaxis platforms. Importantly, we find that ST2 maintains its prognostic capacity in the increasingly popular PT-Cy group. This supplements what we previously reported in the haploidentical and matched unrelated donor PT-Cy setting (Kanakry, Haematologica 2017). Biomarker combination ST2 + CXCL-9 showed improved AUC over ST2 alone, and the composite D14+30 timepoint may have additive utility over a single timepoint. While the optimal time for biomarker collection is unknown, our data suggests D14 had value for ATG-treated patients, whereas D30 may be more important in the PT-Cy-treated group. A validation cohort is being assembled for PT-Cy based MMUD HCT.
Disclosures
Wang:Kite: Consultancy; Sanofi: Consultancy. Jimenez Jimenez:Abbvie: Research Funding. Paczesny:Indiana University Research and Technology Corporation: Patents & Royalties: Biomarkers and assays to detect chronic graft versus host disease (U.S. Patent 10,571,478 B2).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal